Innovative Approaches for Treating Complex Diseases
We are advancing Cellular Nanoparticle programs that address critical unmet needs.
Discovery
Preclinical
Phase 1
Phase 2
CTI-005
Red Blood Cell Nanosponge
Infectious Disease: MRSA Pneumonia
CTI-111
Macrophage Nanosponge
Inflammation: Sepsis
CTI-005
Red Blood Cell Nanosponge
Autoimmune: Autoimmune Hemolytic Anemia
Expansion Programs
Red Blood Cell Nanosponge
Macrophage Nanosponge
Biodefense, Emerging Viruses, Platform Expansion
CTI-005
CTI-005 is for the treatment of Methicillin Resistance Staphylococcus Aureus (MRSA) Pneumonia and has been granted IND allowance from the U.S. FDA.
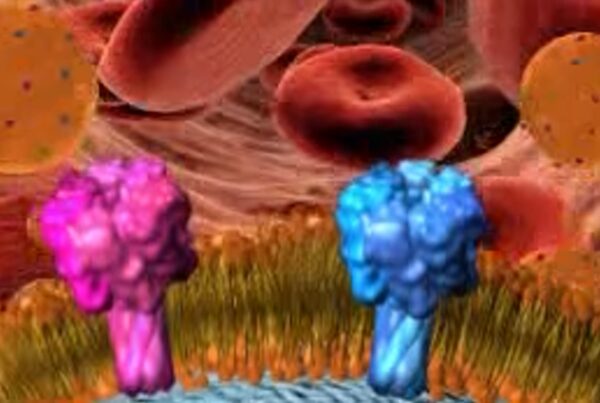
At Cellics, one red blood cell is more than merely a cell, but thousands of RBC-NS. Our potent RBC-NS has the membrane lipids and receptors that a normal red blood cell has, however, the high surface-volume ratio of the RBC-NS allows it to act as a decoy. This characteristic allows the RBC-NS to bind and neutralize toxins, auto-antibodies, and bacterial infections.
One of the bacterial infections that RBC-NS can address is Methicillin Resistance Staphylococcus Aureus (MRSA). The pore-forming toxins emitted by MRSA can be effectively absorbed and neutralized by RBC-NS. Cellics’ drug candidate CTI-005 is for the treatment of MRSA Pneumonia and has received IND allowance to begin a Phase 1b/2a trial in patients. This important milestone validates and de-risks the Cellular Nanosponge platform.
CTI-111
Cellics’ drug candidate CTI-111 is for the treatment of sepsis and has received funding from CARB-X.
Macrophages are the foundation of our immune systems. They surround and kill microorganisms, remove dead cells, and can even stimulate the action of other immune system cells. When macrophages are activated by foreign microorganisms such as bacteria and viruses that enter the human body, they release molecules known as cytokines, which can activate more macrophages and stimulate further immune response and cause inflammation. Severe viral or bacterial infections could cause macrophages to over-respond and cause serious systemic inflammation such as cytokine storm or sepsis.
We craft tens of thousands of MΦ-NS from just one macrophage. The MΦ-NS is a powerful advancement in nanomedicine, with versatile applications. On one hand, the MΦ-NS can effectively absorb and neutralize inflammatory cytokines and control inflammation, used to treat diseases such as Sepsis and Cytokine Release Syndrome (CRS). On the other hand, the Macrophage Nanosponge can also be made into oral, topical, or intraarticular formulations to treat inflammatory diseases such as Inflammatory Bowel Disease and Arthritis.
Cellics’ CTI-111 is being advanced for the treatment of sepsis and has received funding from CARB-X. This important partnership with CARB-X enables Cellics to advance its highly versatile platform to treat not only sepsis but other inflammatory, autoimmune, or infectious diseases with high unmet medical needs.
Pipeline Expansion
Expansion of the Cellics Nanosponge pipeline also includes leveraging CNP™ technology to advance programs for emerging viruses, autoimmune hemolytic anemia and biodefense/countermeasures.
We Strive to Advance Transformative Therapies for Serious Diseases
Cellics seeks to broadly apply its proprietary CNP technology to better treat and prevent serious diseases with high unmet medical needs. Our team includes creative, resourceful, and well-connected life sciences industry veterans.